Embark on an intellectual journey into the realm of electron configuration and orbital diagrams, where the intricacies of atomic structure unfold. Electron Configuration and Orbital Diagram Worksheet Answers unveils the secrets of electron arrangements and their profound impact on the properties of elements.
Prepare to unravel the mysteries of chemistry with this comprehensive guide.
Delve into the fundamental principles of electron configuration, deciphering the rules that govern the distribution of electrons within atomic orbitals. Master the art of constructing orbital diagrams, visualizing the spatial arrangement of electrons. Engage with a meticulously crafted worksheet, testing your understanding and reinforcing key concepts.
Electron Configuration and Orbital Diagrams: Electron Configuration And Orbital Diagram Worksheet Answers
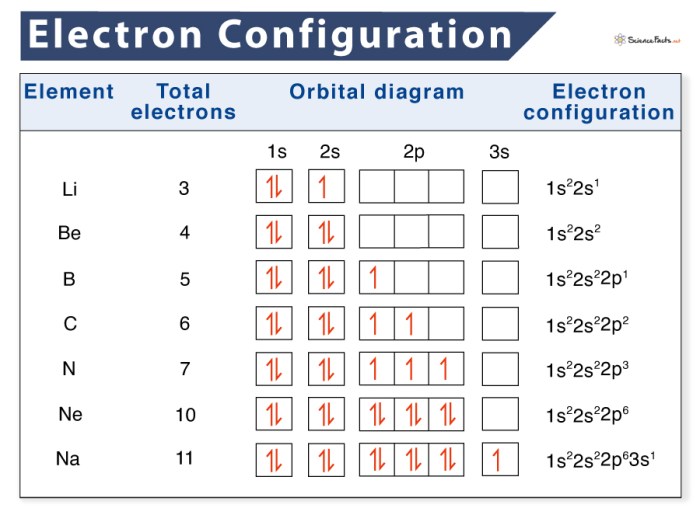
Electron configuration and orbital diagrams are two important concepts in chemistry that describe the arrangement of electrons around the nucleus of an atom. Electron configuration is a notation that shows the number of electrons in each energy level and subshell of an atom, while an orbital diagram is a visual representation of the electron configuration.
Electron Configuration
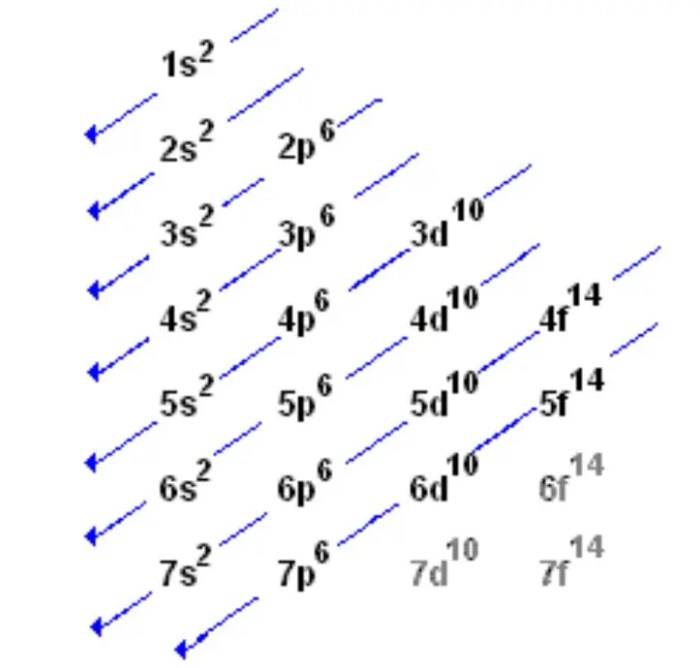
The electron configuration of an element is determined by the number of protons in its nucleus. The number of protons determines the number of electrons that the atom has, and the arrangement of these electrons determines the element’s properties. The rules for writing electron configurations are as follows:
- Electrons are filled into the lowest energy levels first.
- Each energy level can hold a maximum of 2n2electrons, where n is the energy level.
- Each subshell can hold a maximum of 2 electrons.
- The order of the subshells is: s, p, d, f.
For example, the electron configuration of oxygen is 1s 22s 22p 4. This means that oxygen has two electrons in the 1s subshell, two electrons in the 2s subshell, and four electrons in the 2p subshell.
Orbital Diagrams, Electron configuration and orbital diagram worksheet answers
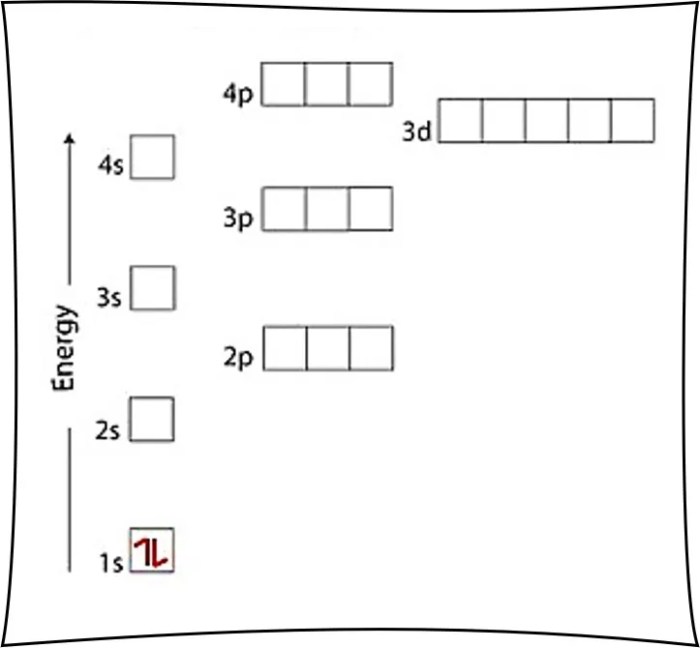
An orbital diagram is a visual representation of the electron configuration of an atom. Each orbital is represented by a box, and the number of electrons in each orbital is indicated by the number of arrows in the box. The arrows represent the spin of the electrons, with up arrows representing electrons with spin up and down arrows representing electrons with spin down.For
example, the orbital diagram of oxygen is:
↑↓ | | 1s | | 2s | | ↑↓ ↑↓ | | | 2p | | | | | |
This orbital diagram shows that oxygen has two electrons in the 1s orbital, two electrons in the 2s orbital, and four electrons in the 2p orbitals.
The two electrons in the 2p orbitals have opposite spins, which is indicated by the up and down arrows.
Expert Answers
What is electron configuration?
Electron configuration refers to the distribution of electrons within the atomic orbitals of an element.
How do I draw an orbital diagram?
Orbital diagrams depict the arrangement of electrons in atomic orbitals using arrows. Follow the rules of electron configuration and Hund’s rule to accurately represent the electron distribution.
What are the limitations of electron configuration and orbital diagrams?
While these models provide valuable insights, they do not account for electron correlation or the dynamic nature of electron behavior.