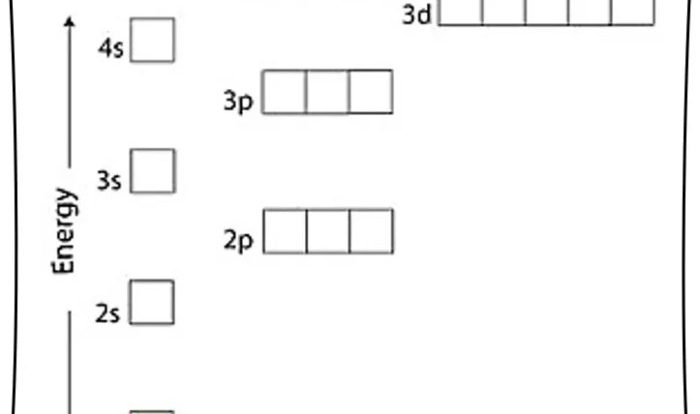
The ACS Chem 2 Formula Sheet is an indispensable tool for students navigating the complexities of chemistry. It condenses a wealth of essential formulas and concepts into a single, organized resource, empowering learners to tackle inorganic, organic, physical chemistry, and various applications with confidence.
Delving into the formula sheet’s sections, you’ll encounter a comprehensive guide to inorganic chemistry, including formulas for common compounds and explanations of oxidation states, coordination complexes, and acid-base reactions. Organic chemistry enthusiasts will appreciate the inclusion of functional group formulas, reaction mechanisms, and stereochemistry principles.
Basic Concepts
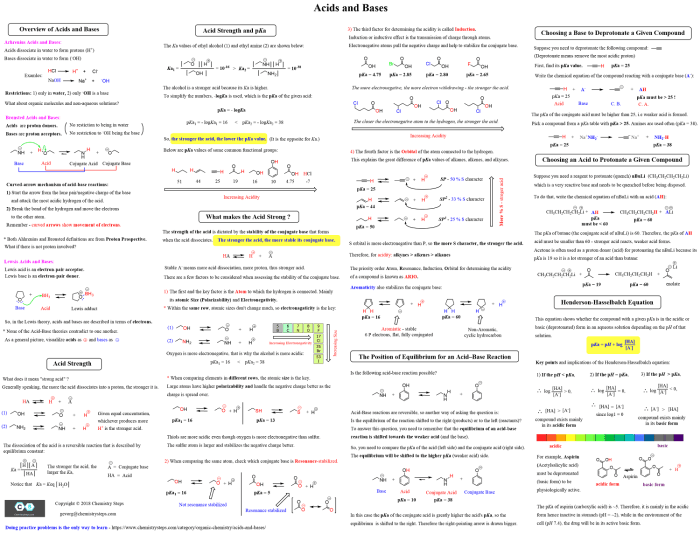
The ACS Chem 2 Formula Sheet is a comprehensive resource that provides essential formulas and concepts for success in ACS Chemistry
- It is organized into several key sections, including:
- Thermodynamics
- Kinetics
- Equilibrium
- Electrochemistry
- Quantum Chemistry
Understanding the formulas and concepts presented in the formula sheet is crucial for success in ACS Chemistry 2. These formulas and concepts provide the foundation for understanding chemical reactions, predicting their outcomes, and solving problems related to chemical systems.
Purpose of the Formula Sheet
The ACS Chem 2 Formula Sheet serves multiple purposes:
- Provides a concise and organized summary of key formulas and concepts.
- Facilitates quick reference and retrieval of information during exams.
- Helps students identify the most important formulas and concepts to focus on.
- Reinforces understanding of the underlying principles of chemistry.
Importance of Understanding the Formulas and Concepts
Understanding the formulas and concepts in the formula sheet is essential because:
- They form the basis for solving problems and making predictions in chemical systems.
- They allow students to connect theoretical concepts to real-world applications.
- They provide a framework for organizing and understanding the vast amount of information in ACS Chemistry 2.
Inorganic Chemistry
Inorganic chemistry explores the properties and behavior of inorganic compounds, which lack carbon-hydrogen bonds. This field encompasses a vast array of substances, from simple ions to complex coordination compounds, and plays a crucial role in various industries and applications.
Formulas and Properties of Common Inorganic Compounds
The formula sheet provides a comprehensive list of common inorganic compounds, along with their formulas and key properties. These compounds include acids, bases, salts, and oxides. Understanding their properties is essential for predicting their reactivity and behavior in chemical reactions.
- Acids: Acids are substances that donate protons (H+ ions) in aqueous solutions. They typically have a sour taste and react with bases to form salts and water.
- Bases: Bases are substances that accept protons (H+ ions) in aqueous solutions. They typically have a bitter taste and react with acids to form salts and water.
- Salts: Salts are ionic compounds formed by the neutralization reaction between an acid and a base. They are typically soluble in water and conduct electricity.
- Oxides: Oxides are compounds that contain oxygen atoms bonded to other elements. They exhibit a wide range of properties, depending on the element involved.
Oxidation States, Acs chem 2 formula sheet
Oxidation states describe the hypothetical charge of an atom in a compound. They are used to determine the oxidation and reduction reactions that a compound can undergo. The formula sheet provides rules for assigning oxidation states to different elements.
Coordination Complexes
Coordination complexes are compounds that contain a metal ion surrounded by ligands. Ligands are molecules or ions that donate electrons to the metal ion. The formula sheet lists common ligands and their coordination modes.
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons between acids and bases. The formula sheet provides equations for various acid-base reactions, including neutralization reactions, acid-base titrations, and buffer solutions.
Example: Using the Formula Sheet to Solve Inorganic Chemistry Problems
The formula sheet can be used to solve a variety of inorganic chemistry problems. For example, it can be used to:
- Identify the formula of a compound given its name.
- Predict the products of a chemical reaction.
- Calculate the pH of a solution.
- Determine the oxidation state of an atom.
Organic Chemistry: Acs Chem 2 Formula Sheet
Organic chemistry is the study of carbon-containing compounds, which are the basis of all living matter. It is a vast and complex field, but some of the most important concepts include the formulas and nomenclature of common organic functional groups, the principles of organic reaction mechanisms and stereochemistry, and how to utilize the formula sheet to predict the products of organic reactions.
Common Organic Functional Groups
Organic functional groups are atoms or groups of atoms that are attached to a carbon atom and give the molecule its characteristic chemical properties. Some of the most common functional groups include:
- Alkanes: CnH2n+2 (e.g., methane, ethane)
- Alkenes: CnH2n (e.g., ethylene, propene)
- Alkynes: CnH2n-2 (e.g., acetylene, propyne)
- Alcohols: ROH (e.g., methanol, ethanol)
- Aldehydes: RCHO (e.g., formaldehyde, acetaldehyde)
- Ketones: RCOR’ (e.g., acetone, butanone)
- Carboxylic acids: RCOOH (e.g., formic acid, acetic acid)
- Esters: RCOOR’ (e.g., methyl acetate, ethyl benzoate)
- Amides: RCONH2 (e.g., acetamide, benzamide)
- Amines: RNH2 (e.g., methylamine, ethylamine)
Organic Reaction Mechanisms
Organic reaction mechanisms are the step-by-step processes by which organic reactions occur. These mechanisms can be complex, but they can be understood by considering the following principles:
- The curly arrow mechanism shows the movement of electrons during a reaction.
- The rate-determining step is the slowest step in a reaction and determines the overall rate of the reaction.
- The Hammond postulate states that the transition state of a reaction resembles the structure of the product.
Stereochemistry
Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules. Stereochemistry is important in organic chemistry because it can affect the physical and chemical properties of molecules.
- Enantiomers are molecules that are mirror images of each other.
- Diastereomers are molecules that are not mirror images of each other but have the same molecular formula.
- The Cahn-Ingold-Prelog (CIP) system is used to assign absolute configurations to chiral molecules.
Predicting the Products of Organic Reactions
The formula sheet can be used to predict the products of organic reactions by considering the following steps:
- Identify the functional groups in the reactants.
- Determine the type of reaction that is likely to occur.
- Use the formula sheet to find the products of the reaction.
Physical Chemistry

Physical chemistry delves into the macroscopic and microscopic properties of matter and how these properties are affected by changes in energy. It encompasses three fundamental concepts: thermodynamics, kinetics, and equilibrium.
Thermodynamics
Thermodynamics examines the relationships between heat, work, and energy in chemical systems.
- Enthalpy (H) measures the heat content of a system.
- Entropy (S) quantifies the randomness or disorder of a system.
- Gibbs Free Energy (G) determines the spontaneity of a reaction.
- ΔG = ΔH – TΔS
- A negative ΔG indicates a spontaneous reaction.
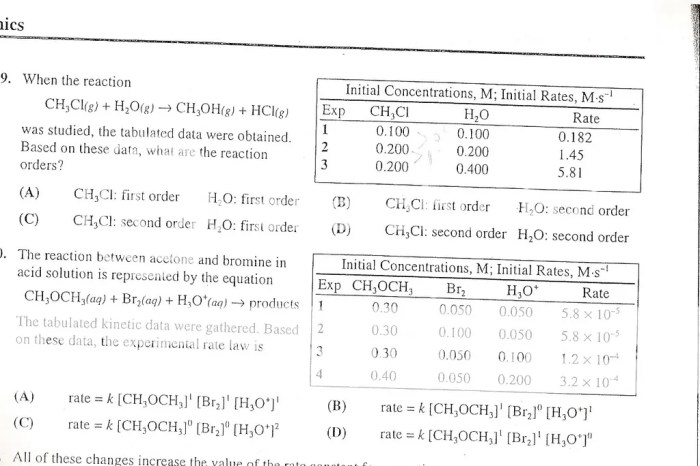
Kinetics
Kinetics focuses on the rates of chemical reactions.
Rate Law
rate = k[A]^m[B]^n
- k is the rate constant.
- [A] and [B] are the concentrations of reactants.
- m and n are the reaction orders.
Arrhenius Equation
k = Ae^(-Ea/RT)
- A is the pre-exponential factor.
- Ea is the activation energy.
- R is the gas constant.
- T is the temperature in Kelvin.
Equilibrium
Equilibrium occurs when the forward and reverse reaction rates are equal.
Equilibrium Constant (K)
K = [products]/[reactants]
- K is a constant for a given reaction at a specific temperature.
- A large K value indicates a reaction that favors products.
Le Chatelier’s Principle
Changes in reaction conditions shift the equilibrium to counteract the change.
While the ACS Chem 2 formula sheet provides essential information for chemistry students, it’s important to remember that practice makes perfect. To enhance your understanding, consider taking the Accu Chek Inform II Quiz to test your knowledge and identify areas where you may need further review.
This quiz will not only reinforce the concepts covered in the formula sheet but also prepare you for upcoming exams.
Applications
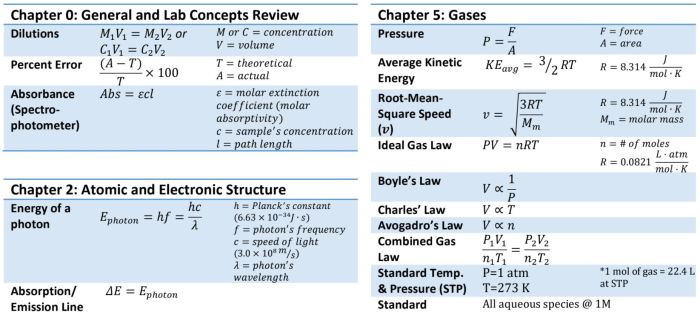
The ACS Chem 2 Formula Sheet is a versatile tool that finds applications in diverse fields, offering a concise and comprehensive reference for chemical concepts and equations.
In research, the formula sheet serves as a quick guide for complex calculations and theoretical derivations. It aids scientists in exploring chemical phenomena, developing new methodologies, and advancing scientific knowledge.
Industry
Within the chemical industry, the formula sheet is an indispensable resource for process optimization, quality control, and product development. It enables engineers and chemists to efficiently design and operate chemical plants, ensuring adherence to safety standards and maximizing productivity.
Everyday Life
The ACS Chem 2 Formula Sheet also has practical applications in everyday life. It provides valuable information for understanding chemical reactions in cooking, cleaning, and household maintenance tasks. It empowers individuals to make informed decisions about the chemicals they use and handle.
FAQ Overview
What is the purpose of the ACS Chem 2 Formula Sheet?
The ACS Chem 2 Formula Sheet provides a concise and organized compilation of essential formulas and concepts across various chemistry disciplines, serving as a valuable resource for students and professionals.
How can I use the formula sheet to solve chemistry problems?
The formula sheet offers a quick reference for formulas and equations related to inorganic, organic, and physical chemistry. By understanding the concepts and applying the formulas appropriately, you can effectively solve chemistry problems.
Is the formula sheet only useful for academic purposes?
No, the ACS Chem 2 Formula Sheet finds applications in various fields beyond academia. It serves as a handy reference for researchers, industry professionals, and anyone requiring a quick refresher on chemistry concepts.